Abstract
Background and Rationale:
Axi-cel is a standard-of-care treatment for relapsed or refractory LBCL after 2 or more lines of systemic therapy. Some patients who would have been ineligible for ZUMA-1 criteria due to comorbidities have been treated with axi-cel in the real-world setting. We interrogated a large registry of patients treated with standard-of-care axi-cel for a definitive report on the impact of age or certain comorbidities on safety and efficacy outcomes after axi-cel infusion.
Methods:
From October 2017 to August 2020, 1500 patients were enrolled in the postapproval safety observational study of axi-cel treated at 79 centers. Patients eligible for the protocol and followed up for at least 6 months with complete data entry by the time of analyses were included. One patient rescinded consent and 1 was deemed ineligible. Patients previously treated with immune effector cell therapy (n=31), with incomplete follow-up reporting (n=92), or alive but last contacted <180 days postinfusion (n=32) were excluded from analysis. Main efficacy outcomes included complete and objective response rates (CR and ORR), duration of response (DOR), progression-free and overall survival (PFS and OS). Safety endpoints of interest included cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). Outcomes were assessed and compared by age and preselected coexisting disease or organ impairment within 3 months prior to the infusion included in the hematopoietic cell transplantation comorbidity index. Multivariate logistic and Cox regression models were used to assess the impact of age or coexistent organ dysfunction on outcomes via odds ratio (OR) or hazard ratio (HR) and their 95% CIs.
Results
A total of 1343 patients were included in the analysis with a median 25.1 months (range, 10.3-42.7 months) for potential follow-up, defined from infusion to data cutoff date, and a median 11.8 months (range, 0.1-38.8 months) for actual follow-up time, defined from infusion to date of death or last contact. Of these, 38% were 65 years or older (median 62 years), 4% had a performance score ≥2, 13% had cardiac comorbidities, 16% had a prior cancer, 9% were obese, 2% had moderate to severe hepatic comorbidities, and 2% had renal comorbidities. Transformed lymphoma, double or triple-hit by fluorescence in situ hybridization, prior autologous transplant and refractory disease were present in 28%, 15%, 27% and 66%, respectively. Bridging therapy was administered in 21% of patients.
Overall, ORR was 74%, (CR 56%), and probabilities of DOR, PFS, and OS at 18 months were 61% (95% CI, 57-65%), 42% (95% CI, 39-45%) and 52% (95% CI, 49-55%), respectively. Overall rates of CRS and ICANS were 83% and 55%, respectively. ORR was 78% (CR 62%, median OS 17.5 [95% CI, 16.0-not evaluated (NE)] months) for patients ≥65 years, ORR 57% (CR 29%; median OS 4.3 [95% CI, 2.5-8.3] months) for patients with hepatic dysfunction, ORR 70% (CR 43%; median OS 8.9 [95% CI, 3.8-NE] months) for patients with renal dysfunction; and ORR 47% (CR 20%, median OS 4 [95% CI, 2.6-6.9] months) for patients with Eastern Cooperative Oncology Group (ECOG) 2 or 3.
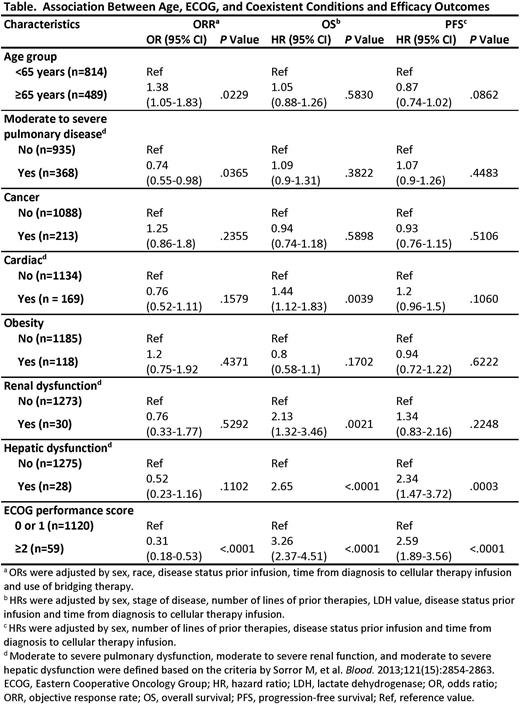
Multivariate analyses indicated that advanced age (≥65 years vs <65 years; OR 1.38; 95% CI, 1.05-1.83), moderate to severe pulmonary disease (yes vs no; OR 0.74; 95% CI, 0.55-0.98), and ECOG (2-3 vs 0-1; OR 0.31, 95% CI, 0.18-0.53) impacted ORR (Table). Age ≥65 years did not have an impact on survival (HR 1.05, 95% CI, 0.88-1.26), although it was associated with CRS (OR 1.42, 95% CI, 1.03-1.96) and ICANS (OR 1.78, 95% CI, 1.39-2.28). Coexistent cardiac disease (HR 1.44, 95% CI, 1.12-1.83), renal disease (HR 2.13, 95% CI, 1.32-3.46) and hepatic dysfunction (HR 2.65, 95% CI, 1.69-4.14) had an impact on OS, but not on response, CRS, and ICANS. ECOG significantly impacted all efficacy outcomes. None of the other comorbidities tested significantly impacted outcomes.
Conclusion: Advanced age (≥65 years) was not associated with worse efficacy outcomes after axi-cel, despite higher rates of CRS and ICANS, which require closer monitoring. Performance status rather than age should be accounted for in patient selection and treatment decisions with axi-cel. Most coexistent organ dysfunctions have no clinically significant impact on axi-cel objective response and safety outcomes.
Locke: Moffitt Cancer Center: Patents & Royalties: field of cellular immunotherapy; Allogene Therapeutics: Consultancy, Other: Scientific Advisory Role, Research Funding; Amgen: Consultancy, Other: Scientific Advisory Role; Bluebird Bio: Consultancy, Other: Scientific Advisory Role; EcoR1: Consultancy; Emerging Therapy Solutions: Consultancy; Gerson Lehrman Group: Consultancy; Iovance Biotherapeutics: Consultancy, Other: Scientific Advisory Role; Takeda: Consultancy, Other; Novartis: Consultancy, Other, Research Funding; Wugen: Consultancy, Other; GammaDelta Therapeutics: Consultancy, Other: Scientific Advisory Role; Umoja: Consultancy, Other; Cellular Biomedicine Group: Consultancy, Other: Scientific Advisory Role; Calibr: Consultancy, Other: Scientific Advisory Role; Cowen: Consultancy; BMS/Celgene: Consultancy, Other: Scientific Advisory Role; Legend Biotech: Consultancy, Other; Kite, a Gilead Company: Consultancy, Other: Scientific Advisory Role, Research Funding; Janssen: Consultancy, Other: Scientific Advisory Role. Jacobson: Axis: Speakers Bureau; Nkarta: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Other: Travel support, Research Funding; AbbVie: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Other: Travel support; Novartis Pharmaceuticals Corporation: Consultancy, Honoraria, Other: Travel support; Precision Biosciences: Consultancy, Honoraria, Other: Travel support; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel support; Lonza: Consultancy, Honoraria, Other: Travel support; Humanigen: Consultancy, Honoraria, Other: Travel support; Clinical Care Options: Speakers Bureau. Ma: Kite, a Gilead Company: Current Employment. Dong: Kite, a Gilead Company: Current Employment. Hu: Kite, a Gilead Company: Research Funding; Novartis: Research Funding; Celgene: Research Funding. Siddiqi: BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Oncternal: Research Funding; Janssen: Speakers Bureau; Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics LLC, an AbbVie Company: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; TG Therapeutics: Research Funding. Ahmed: Seagen: Research Funding; Tessa Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Xencor: Research Funding. Ghobadi: Atara: Consultancy; Wugen: Consultancy; Amgen: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Celgene: Consultancy. Miklos: Pharmacyclics: Patents & Royalties; Kite, a Gilead Company, Amgen, Atara, Wugen, Celgene, Novartis, Juno-Celgene-Bristol Myers Squibb, Allogene, Precision Bioscience, Adicet, Pharmacyclics, Janssen, Takeda, Adaptive Biotechnologies and Miltenyi Biotechnologies: Consultancy; Pharmacyclics, Amgen, Kite, a Gilead Company, Novartis, Roche, Genentech, Becton Dickinson, Isoplexis, Miltenyi, Juno-Celgene-Bristol Myers Squibb, Allogene, Precision Biosciences, Adicet, Adaptive Biotechnologies: Research Funding; Adaptive Biotechnologies, Novartis, Juno/Celgene-BMS, Kite, a Gilead Company, Pharmacyclics-AbbVie, Janssen, Pharmacyclics, AlloGene, Precision Bioscience, Miltenyi Biotech, Adicet, Takeda: Membership on an entity's Board of Directors or advisory committees. Lin: Kite, a Gilead Company: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Legend: Consultancy; Vineti: Consultancy; Sorrento: Consultancy; Gamida Cell: Consultancy; Janssen: Consultancy, Research Funding; Juno: Consultancy; Bluebird Bio: Consultancy, Research Funding; Novartis: Consultancy; Merck: Research Funding; Takeda: Research Funding. Perales: Bristol-Myers Squibb: Honoraria; Takeda: Honoraria; Nektar Therapeutics: Honoraria, Other; Miltenyi Biotec: Honoraria, Other; MorphoSys: Honoraria; NexImmune: Honoraria; Sellas Life Sciences: Honoraria; Cidara: Honoraria; Merck: Honoraria; Omeros: Honoraria; Servier: Honoraria; Celgene: Honoraria; Novartis: Honoraria, Other; Kite/Gilead: Honoraria, Other; Incyte: Honoraria, Other; Medigene: Honoraria; Karyopharm: Honoraria; Equilium: Honoraria. Lunning: Daiichi-Sankyo: Consultancy; TG Therapeutics: Consultancy; Kite, a Gilead Company: Consultancy; ADC Therapeutics: Consultancy; Spectrum: Consultancy; Beigene: Consultancy; Celgene, a Bristol Myers Squibb Co.: Consultancy; Legend: Consultancy; AbbVie: Consultancy; AstraZeneca: Consultancy; Morphosys: Consultancy; Verastem: Consultancy; Novartis: Consultancy; Myeloid Therapeutics: Consultancy; Janssen: Consultancy; Acrotech: Consultancy; Kyowa Kirin: Consultancy; Karyopharm: Consultancy. Hill: Gentenech: Consultancy, Honoraria, Research Funding; Incyte/Morphysis: Consultancy, Honoraria, Research Funding; Beigene: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel Support, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding; AstraZenica: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Epizyme: Consultancy, Honoraria; Celgene (BMS): Consultancy, Honoraria, Research Funding. Nikiforow: Kite/Gilead: Other: ad HOC Advisory Boards; Novartis: Other: ad Hoc Advisory Boards; Iovance: Other: ad Hoc Advisory Boards; Glaxo Smith Kline (GSK): Other: ad Hoc Advisory Boards. Xu: Gilead Sciences: Other: stock or other ownership ; Kite, A Gilead Company: Current Employment. Pasquini: Bristol Myers Squibb: Consultancy, Research Funding; Novartis: Research Funding; Kite Pharma: Research Funding; GlaxoSmithKline: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal